Base mediated green synthesis of enantiopure 2-C-spiro-glycosyl-3-nitrochromenes†
Abstract
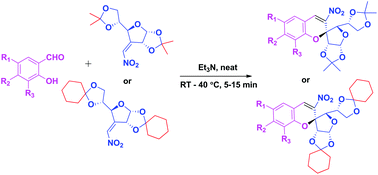
A novel green synthetic methodology has been developed to obtain enantiopure (2S)-2-C-spiro-glycosyl-3-nitrochromenes following the oxa-Michael–aldol condensation reaction of sugar derived 3-C-vinyl nitro olefins with substituted salicylaldehydes using Et3N as a base under neat conditions at rt–40 °C. The stereochemistry of the product is confirmed by a single crystal X-ray study. Several advantages are associated with this protocol such as cost effectiveness, easy accessibility, short reaction time, high yields, wide substrate scope and high enantiopurity.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...