Stereoselective C-terminal peptide elongation from Chan–Lam–Evans reaction generated isopropenyl esters†
Abstract
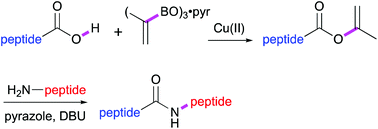
C-Terminal dipeptide isopropenyl esters were synthesised by a Cu(II)-mediated Chan–Lam–Evans enol esterification of peptide carboxylic acids and isoprenyl boroxine. These shelf stable peptide esters could be coupled stereoselectively with a variety of amino acid and dipeptide nucleophiles in high yield and purity in the presence of pyrazole/DBU as the catalyst.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...