Identification of a lysine 4-hydroxylase from the glidobactin biosynthesis and evaluation of its biocatalytic potential†
Abstract
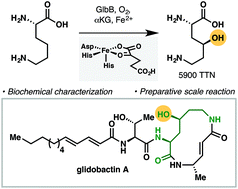
We present the functional characterization of GlbB, a lysine 4-hydroxylase from the glidobactin biosynthetic gene cluster. Despite its narrow substrate specificity, GlbB is able to catalyze the hydroxylation of L-lysine with excellent total turnover number and complete regio- and diastereoselectivity. The synthetic utility of GlbB is illustrated by its use in the efficient preparation of a key dipeptide fragment of glidobactin.
- This article is part of the themed collections: Chemical Biology in OBC and New Talent


 Please wait while we load your content...
Please wait while we load your content...