Palladium-catalyzed salt-free double decarboxylative aryl allylation†
Abstract
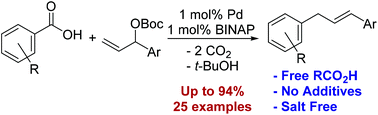
This report describes a palladium-catalyzed decarboxylative aryl allylation between unactivated benzoic acids and allylic carbonates. This transformation successfully couples a variety of carbonates and benzoic acids in good yield (up to 94%) using 1 mol% palladium. This salt free allyl-arylation proceeds without added base, copper, or silver. The only stoichiometric byproducts are carbon dioxide and tert-butanol.
- This article is part of the themed collection: New Talent


 Please wait while we load your content...
Please wait while we load your content...