Organocatalytic asymmetric Michael/hemiketalization/acyl transfer reaction of 1,3-propanediones with (E)-2-(2-nitrovinyl)phenols†
Abstract
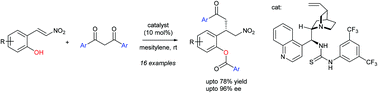
An organocatalytic asymmetric cascade Michael/hemiketalization/acyl transfer reaction between (E)-2-(2-nitrovinyl)phenols and 1,3-propanediones is disclosed. A cinchona alkaloid derived bifunctional thiourea catalyst was found to be the most effective for this reaction and provided the desired products in moderate to good yields with good to high enantioselectivities.
- This article is part of the themed collections: Catalysis & biocatalysis in OBC and New Talent


 Please wait while we load your content...
Please wait while we load your content...