A bisubstrate reagent orchestrating adenosine triphosphate and l-tyrosine and making tyrosyl adenylate: partial mimicking of tyrosyl-tRNA synthetase†
Abstract
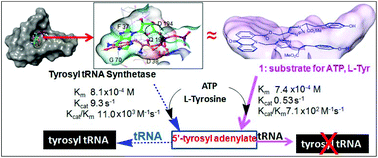
We report the development of a bisubstrate reagent that, similar to tyrosyl t-RNA synthetase (TyrTS), provides a surface for ATP and L-Tyr to render a pseudo-intramolecular reaction forming 5′-tyrosyl adenylate (tyrAd). The presence of the reagent in solution with TyrTS marred the enzymatic reaction and, noticeably, tyrAd formed under the catalytic mode of the biomodel reagent was not picked up by TyrTS and hence was not transferred to tRNA. A potential application of this reagent, which doesn't allow the formation of tyrosyl tRNA, may lie in an emerging therapeutic targeting the translation machinery of cells without inhibiting the normal workings of enzymes.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...