Atom-economical selenation of electron-rich arenes and phosphonates with molecular oxygen at room temperature†
Abstract
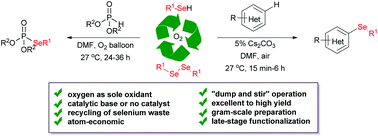
Organoselenium and selenophosphorus compounds are ubiquitously found in biologically active compounds, agrochemicals, functionalized materials etc. Although selenium is a micronutrient and an essential trace element, its contamination/consumption in higher concentrations is extremely dangerous. However, most of the previous selenation reactions generate toxic selenium waste as a by-product. Thus development of green synthetic protocols of these compounds is in high demand. We report herein a mild base-catalyzed cross-dehydrogenative coupling (CDC) between electron-rich arenes and phenylselenol to afford 3-selenylindole or selenylated phenols under air at room temperature. Interestingly, in the presence of a base and oxygen, the phenylselenol is converted into the diphenyldiselenide and provides almost quantitative yield. Similarly, a mild synthesis of selenophosphates was also achieved from the corresponding diorganyldiselenide or phenylselenols and nucleophilic phosphonates in a “dump and stir” manner under an oxygen balloon without a base or catalyst. From the preliminary mechanistic studies for selenation of indoles and phosphonates with TEMPO and EPR of the reaction mixture, it was evident that the reaction proceeds through the anionic pathway, which is in sharp contrast to the previous literature. The present reactions proceed smoothly under the mild conditions, furnishing high to almost quantitative yields in several cases. The reaction is easily scaled up to gram scale and has been demonstrated for the synthesis of an anti-HIV zidovudine (AZT) analogue.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...