Rh-Catalyzed aminative dearomatization of 2-naphthols†
Abstract
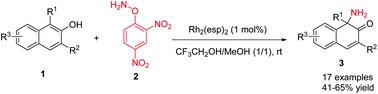
A direct aminative dearomatization of 2-naphthols was achieved. In the presence of 1 mol% Rh2(esp)2 and 3 equivalents of O-(2,4-dinitrophenyl)hydroxylamine (DPH) as readily available aminating reagents, the reactions of 2-naphthols afforded unprotected α-amino-β-naphthalenones in good yields under mild reaction conditions. The conditions were compatible with gram-scale reaction, and the product could undergo diverse transformations.


 Please wait while we load your content...
Please wait while we load your content...