Chiral isoxazolidine-mediated stereoselective umpolung α-phenylation of methyl ketones†
Abstract
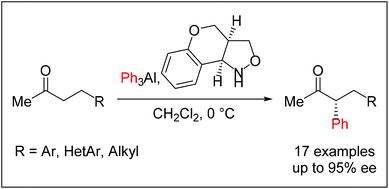
An effective asymmetric α-phenylation of methyl ketones with triphenylaluminium in the presence of (+)-benzopyranoisoxazolidine has been developed. The reaction proceeds via the in situ formation of a chiral N-alkoxyenamine and the subsequent diastereoselective nucleophilic phenylation to provide α-phenylated products in moderate to good yields, with high enantioselectivities.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...