A new synthetic route to 5,6,11,12-tetraarylethynyltetracenes†
Abstract
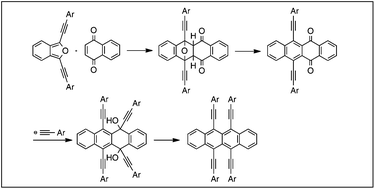
A new synthetic route to 5,6,11,12-tetrakis(arylethynyl)tetracenes, π-extended rubrenes, was developed via [4 + 2] cycloadditions of dialkynylisobenzofuran and 1,4-naphthoquinone. Introduction of arylethynyl groups by double nucleophilic additions to tetracenequinone gave sterically congested (arylethynyl)tetracenes after reductive aromatization. The photophysical properties of the newly prepared π-conjugated molecules are also evaluated.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...
