Asymmetric hydrogenation of imines with chiral alkene-derived boron Lewis acids†
Abstract
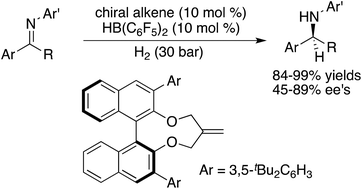
With the aim of developing easily accessible chiral Lewis acids for asymmetric hydrogenation, a variety of binaphthyl-based chiral alkenes were prepared in one step from the corresponding diols. Using the in situ generated chiral boron Lewis acids through the hydroboration of chiral alkenes with Piers’ borane, metal-free asymmetric hydrogenations of imines were realized to furnish the desired amine products in high yields with up to 89% ee.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...