Donor- and acceptor-enynals/enynones
Abstract
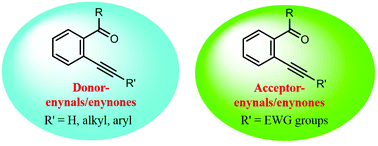
Enynals and enynones have emerged as focal substrates in organic synthesis in view of their structure diversity, high reactivity and intermediate variety. Transition metal- or Lewis acid-promoted protocols starting from enynals/enynones could provide efficient and direct access to functionalized homo- or heterocyclic compounds. In consideration of the different electronic properties of substituents on the alkyne group of enynals/enynones, they are classified into two kinds in this review, namely donor-enynals/enynones with non-electron-withdrawing substituents and acceptor-enynals/enynones with electron-withdrawing moieties. Herein we mainly introduced three kinds of transformations based on benzo-fused donor- and acceptor-enynals/enynones, including their reactions with alkenes, alkynes, and H2O, respectively.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...