Synthesis of functionalized cyclopentenes through allenic ketone-based multicomponent reactions†
Abstract
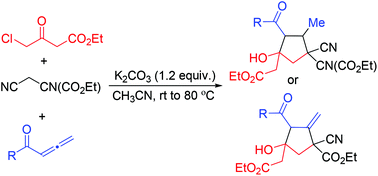
A novel and efficient synthesis of diversely functionalized cyclopentene derivatives through the multicomponent reactions of 1,2-allenic ketones with 4-chloroacetoacetate and malononitrile/cyanoacetate under mild and metal-free conditions is presented. Mechanistically, the formation of title compounds involves a cascade process including nucleophilic substitution, Michael addition and intramolecular aldol type reaction. Interestingly, when 1-phenyl allenic ketones bearing electron-donating groups on the phenyl ring were reacted with 4-chloroacetoacetate and cyanoacetate, methylenecyclo-pentanes, the regioisomer of cyclopentenes, were formed with good selectivity and high efficiency.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...