Rh(iii)-Catalyzed dual C–H functionalization of 3-(1H-indol-3-yl)-3-oxopropanenitriles with sulfoxonium ylides or diazo compounds toward polysubstituted carbazoles†
Abstract
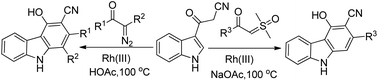
A rhodium-catalyzed annulation of 3-(1H-indol-3-yl)-3-oxopropanenitriles with sulfoxonium ylides or diazo compounds has been developed, leading to a series of polysubstituted carbazoles in moderate to good yields. This procedure proceeded with formal Rh(III)-catalyzed (4 + 2) cycloaddition, with the functionalization of 2-C–H bonds of indole in a step-economical procedure. Additionally, this reaction could also be conducted under acidic conditions when diazo compounds were employed as the reaction partners, which was a complement to the annulation of sulfoxonium ylides under weak basic conditions.


 Please wait while we load your content...
Please wait while we load your content...