Development of photoswitchable inhibitors for β-galactosidase†
Abstract
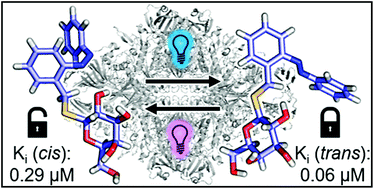
Azobenzenes are of particular interest as a photochromic scaffold for biological applications because of their high fatigue resistance, their large geometrical change between extended (trans) and bent (cis) isomer, and their diverse synthetic accessibility. Despite their wide-spread use, there is no reported photochromic inhibitor of the well-investigated enzyme β-galactosidase, which plays an important role for biochemistry and single molecule studies. Herein, we report the synthesis of photochromic competitive β-galactosidase inhibitors based on the molecular structure of 2-phenylethyl β-D-thiogalactoside (PETG) and 1-amino-1-deoxy-β-D-galactose (β-D-galactosylamine). The thermally highly stable PETG-based azobenzenes show excellent photochromic properties in polar solvents and moderate to high photostationary states (PSS). The optimized compound 37 is a strong competitive inhibitior of β-galactosidase from Escherichia coli and its inhibition constant (Ki) changes between 60 nM and 290 nM upon irradiation with light. Additional docking experiments supported the observed structure–activity relationship.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...