Suzuki–Miyaura coupling of unstrained ketones via chelation-assisted C–C bond cleavage†
Abstract
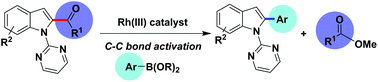
Herein, we report that unstrained ketones can be efficiently employed as electrophiles in Suzuki–Miyaura reactions via catalytic activation of unstrained C–C bonds assist by an N-containing directing group. A wide range of aromatic ketones directly coupled with boronic ester with excellent functional group tolerance. This strategy provides an alternative and versatile approach to constructing biaryls from unstrained ketones.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...