Bioactive polycyclic polyprenylated acylphloroglucinols from Hypericum perforatum†
Abstract
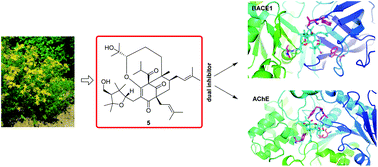
Fifteen new polycyclic polyprenylated acylphloroglucinols (PPAPs), hyperforatones A–O (1–15), along with 3 structurally related analogues (16–18), were isolated from the stems and leaves of Hypericum perforatum. Their structures and absolute configurations were established by a combination of NMR spectroscopic analyses, experimental and calculated electronic circular dichroism (ECD), modified Mosher's methods, Rh2(OCOCF3)4- and [Mo2(OAc)4]-induced ECD, X-ray crystallography, and the assistance of quantum chemical predictions (QCP) of 13C NMR chemical shifts. Compound 5 was found to be the first PPAP decorated by a rare 2,2,4,4,5-(pentamethyltetrahydrofuran-3-yl)methanol moiety and an oxepane ring. Furthermore, the isolates were screened for their acetylcholinesterase (AChE) and β-site amyloid precursor protein cleaving enzyme 1 (BACE1) inhibitory activities. Compounds 5, 10, 11, and 15 showed desirable AChE inhibitory activities (IC50 6.9–9.2 μM) and simultaneously inhibited BACE1 (at a concentration of 5 μM) with inhibition rates of 50.3%, 34.3%, 47.2%, and 34.6%, respectively. Interestingly, compound 5 showed the most balanced inhibitory activities against both AChE and BACE1 of all the tested compounds, which means that 5 could serve as the first valuable dual-targeted PPAP for the treatment of Alzheimer's disease. Preliminary molecular docking studies of 5 with BACE1 and AChE were also performed.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...