Mutated variants of squalene-hopene cyclase: enzymatic syntheses of triterpenes bearing oxygen-bridged monocycles and a new 6,6,6,6,6-fusded pentacyclic scaffold, named neogammacerane, from 2,3-oxidosqualene†
Abstract
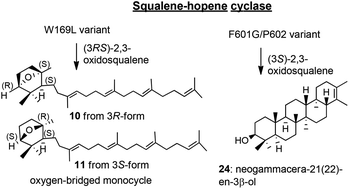
Squalene-hopene cyclase (SHC) catalyzes the conversion of acyclic squalene molecule into a 6,6,6,6,5-fused pentacyclic hopene and hopanol. SHC is also able to convert (3S)-2,3-oxidosqualene into 3β-hydroxyhopene and 3β-hydroxyhopanol and can generate 3α-hydroxyhopene and 3α-hydroxyhopanol from (3R)-2,3-oxidosqualene. Functional analyses of active site residues toward the squalene cyclization reaction have been extensively reported, but investigations of the cyclization reactions of (3R,S)-oxidosqualene by SHC have rarely been reported. The cyclization reactions of oxidosqualene with W169X, G600F/F601G and F601G/P602F were examined. The variants of the W169L generated new triterpene skeletons possessing a 7-oxabicyclo[2.2.1]heptane moiety (oxygen-bridged monocycle) with (1S,2S,4R)- and (1R,2S,4S)-stereochemistry, which were produced from (3R)- and (3S)-oxidosqualenes, respectively. The F601G/P602F double mutant also furnished a novel triterpene, named neogammacer-21(22)-en-3β-ol, consisting of a 6,6,6,6,6-fused pentacyclic system, in which Me-29 at C-22 of the gammacerane skeleton migrated to C-21. We propose to name this novel scaffold neogammacerane. The formation mechanisms of the enzymatic products from 2,3-oxidosqualene are discussed.
- This article is part of the themed collection: Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...