Organic amine-mediated free-radical carbocyclization reactions of 2,2,2-trihalogeno-substituted N-(2-alkynylphenyl)acetamides†
Abstract
An efficient method for the synthesis of 3-halogeno-substituted 4-benzoylquinolin-2-(1H)-ones from N-(2-alkynylphenyl)-substituted trihaloacetamides has been developed, in which organic amines (TNPA and DIEA) act as the electron donors. In this carbocyclization reaction, a new C–C bond formation occurred regioselectively via a 6-exo-dig radical cyclization. A variety of useful functional groups are compatible with the reaction conditions. In this process, readily removable organic amines were employed and no heavy metal catalysts were required.
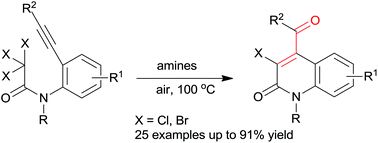
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...