Chemoselective N–H functionalization of indole derivatives via the Reissert-type reaction catalyzed by a chiral phosphoric acid†
Abstract
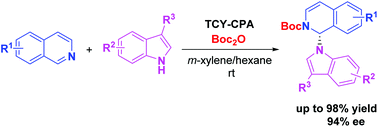
An asymmetric N-alkylation of indole derivatives via the Reissert-type reaction was realized in the presence of a catalytic amount of chiral phosphoric acid. Various enantioenriched indoles with N-1 substituted by 1,2-dihydroisoquinoline could be obtained under mild conditions in good yields and enantioselectivities at room temperature (up to 98% yield, 94% ee). The current method is compatible with gram-scale reaction and the products can undergo versatile chemical transformations.
- This article is part of the themed collections: Synthetic methodology in OBC and Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...