Magnolol dimer-derived fragments as PPARγ-selective probes†
Abstract
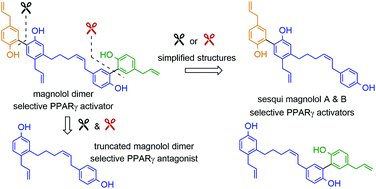
Partial agonists of the transcription factor PPARγ (peroxisome proliferator-activated receptor γ) have shown potential for the treatment of metabolic and inflammatory conditions and novel activators serve as valuable tool and lead compounds. Based on the natural product magnolol (I) and recent structural information of the ligand–target interaction we have previously developed magnolol dimer (II) which has been shown to have enhanced affinity towards PPARγ and improved selectivity over RXRα (retinoid X receptor α), PPARγ's heterodimerization partner. In this contribution we report the synthesis and evaluation of three fragments of the dimeric lead compound by structural simplifications. Sesqui magnolol A and B (III and IV) were found to exhibit comparable activities to magnolol dimer (II) and selectivity over RXRα persisted. Computational studies suggest a common pharmacophore of the distinctive biphenyl motifs. Truncated magnolol dimer (V) on the other hand does not share this feature and was found to act as an antagonist.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...