Nickel catalyzed site selective C–H functionalization of α-aryl-thioamides†
Abstract
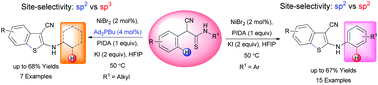
A catalytic site selective intramolecular C–S bond forming reaction is demonstrated for the first time. The C–H bond functionalization of α-aryl-thioacetanilides was efficiently catalyzed by 2 mol% NiBr2, resulting in valuable 2-aminobenzo[b]thiophenes in moderate to good yields. Furthermore, the selective sp2 C–H bond functionalization over sp3 is exemplified.
- This article is part of the themed collections: Synthetic methodology in OBC and Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...