Radical alkylation of para-quinone methides with 4-substituted Hantzsch esters/nitriles via organic photoredox catalysis†
Abstract
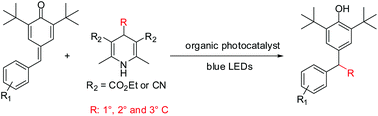
A novel photocatalytic protocol is herein described for the preparation of functionalized phenols via radical alkylation of para-quinone methides under transition-metal-free conditions. The reaction is external oxidant free and performed at ambient temperature upon visible light irradiation, allowing the access to various desired products in satisfactory yields. The readily available 4-alkyl-1,4-dihydropyridines serve as the effective alkyl radical precursors.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...