Unusual anti-inflammatory meroterpenoids from the marine sponge Dactylospongia sp.†
Abstract
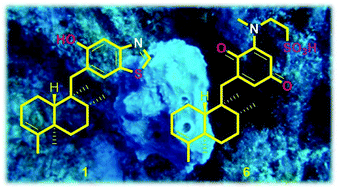
Five new sesquiterpene hydroquinones, dactylospongins A–D (1–4) and 19-O-methylpelorol (10), as well as four new sesquiterpene quinones, melemeleones C–E (6–8) and dysidaminone N (9), were isolated from the marine sponge Dactylospongia sp. collected from the South China Sea, along with five known analogues, ent-melemeleone B (5), pelorol (11), 17-O-acetylavarol (12), 20-O-acetylavarol (13), and 20-O-acetylneoavarol (14). The structures were elucidated by spectroscopic data analyses and comparison with the published NMR data, while the absolute configurations of new structures were assigned by comparison between the experimental and calculated ECD spectra. Dactylospongins A (1) and B (2) are the first sesquiterpenoids with a benzothiazole ring from the marine environment. Anti-inflammatory evaluation showed that 1, 2, 4, 5, 9, and 10 showed potent inhibitory effects on the production of inflammatory cytokines (IL-6, IL-1β, IL-8, and PEG2) in LPS-induced THP-1 cells with IC50 values of 5.1–9.2 μM; however, none of them showed significant effects on the production of MCP-1 and TNF-α. Additionally, 19-O-methylpelorol (10) exhibited cytotoxicity against lung cancer PC-9 cell lines with an IC50 value of 9.2 μM.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...