Synthesis of α-oxygenated β,γ-unsaturated ketones by a catalytic rearrangement strategy†
Abstract
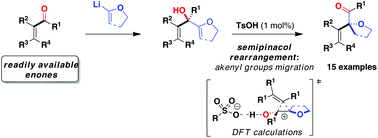
A straightforward two-step entry to α-oxgenated β,γ-unsaturated ketones from readily available α,β-unsaturated ketones is disclosed. It was found that bis(allylic) alcohols undergo a skeletal rearrangement in the presence of 1 mol% of cheap and non-corrosive p-toluenesulfonic acid. Computational studies were conducted to support the mechanism and to rationalise the influence of the catalyst acidity on the product selectivity.


 Please wait while we load your content...
Please wait while we load your content...