Molecular diversity of the domino annulation reaction of 2-aryl-3-nitrochromenes with pivaloylacetonitriles†
Abstract
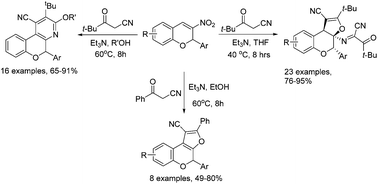
In the presence of triethylamine, the domino annulation reaction of two molecules of pivaloylacetonitrile with one molecule of 2-aryl-3-nitrochromene in tetrahydrofuran resulted in the unprecedented imino-substituted dihydrofuro[2,3-c]chromene derivatives in high yields. More importantly, the above domino reaction in refluxing methanol or ethanol afforded alkoxy-substituted chromeno[3,4-b]pyridines in satisfactory yields. However, a similar reaction of benzoylacetonitrile with 2-aryl-3-nitrochromenes in basic medium gave the expected furo[2,3-c]chromene derivatives in moderate yields.


 Please wait while we load your content...
Please wait while we load your content...