Synthesis of medium-sized (6–7–6) ring compounds by iron-catalyzed dehydrogenative C–H activation/annulation†
Abstract
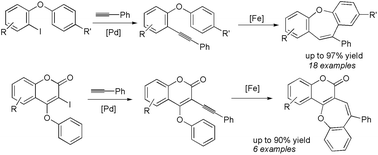
In this report, we have described a FeCl3-catalyzed process involving intramolecular annulation of o-phenoxy diarylacetylenes via hydroarylation to afford a series of biologically potent fused seven-membered (6–7–6) ring compounds under mild reaction conditions. This reaction was believed to proceed through Friedel–Crafts type sequential carbometallation followed by protonation to produce phenyldibenz[b,f]oxepines. This method was also extended to synthesize seven-membered rings that are fused with coumarins.
- This article is part of the themed collection: Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...