Pd-Catalyzed C(sp2)–H aminocarbonylation using the Langlois reagent as a carbonyl source†
Abstract
A Pd-catalyzed C(sp2)–H aminocarbonylation of aryl carboxamides assisted by an N,S-bidentate directing group was developed, in which cheap and stable sodium trifluoromethanesulfinate was first utilized as a carbonyl source. The reaction can be applicable to a wide range of carboxamides with good functional group tolerance and afford isoindole-1,3-diones in moderate to good yields.
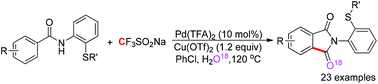
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...