TEMPO promoted direct multi-functionalization of terminal alkynes with 2-oxindoles/benzofuran-2(3H)-one†
Abstract
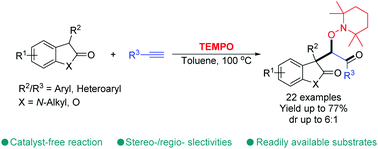
Highly selective and catalyst-free tandem multi-functionalization of terminal alkynes was developed with 2-oxindoles and benzo-furan-2(3H)-one using TEMPO both as a radical promoter and a trapping reagent. This work expands the scope of the radical-cascade addition/trapping process of alkynes for the effective construction of various β-oxyl carbonyls in moderate to good yields.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...