Quinoline–galactose hybrids bind selectively with high affinity to a galectin-8 N-terminal domain†
Abstract
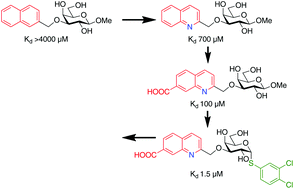
Quinolines, indolizines, and coumarins are well known structural elements in many biologically active molecules. In this report, we have developed straightforward methods to incorporate quinoline, indolizine, and coumarin structures into galactoside derivatives under robust reaction conditions for the discovery of glycomimetic inhibitors of the galectin family of proteins that are involved in immunological and tumor-promoting biological processes. Evaluation of the quinoline, indolizine and coumarin-derivatised galactosides as inhibitors of the human galectin-1, 2, 3, 4N (N-terminal domain), 4C (C-terminal domain), 7, 8N, 8C, 9N, and 9C revealed quinoline derivatives that selectively bound galectin-8N, a galectin with key roles in lymphangiogenesis, tumor progression, and autophagy, with up to nearly 60-fold affinity improvements relative to methyl β-D-galactopyranoside. Molecular dynamics simulations proposed an interaction mode in which Arg59 had moved 2.5 Å and in which an inhibitor carboxylate and quinoline nitrogen formed structure-stabilizing water-mediated hydrogen bonds. The compounds were demonstrated to be non-toxic in an MTT assay with several breast cancer cell lines and one normal cell line. The improved affinity, selectivity, and low cytotoxicity suggest that the quinoline–galactoside derivatives provide an attractive starting point for the development of galectin-8N inhibitors potentially interfering with pathological lymphangiogenesis, autophagy, and tumor progression.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...