Peripheral cyclic β-amino acids balance the stability and edge-protection of β-sandwiches†
Abstract
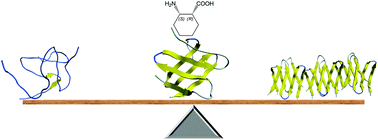
Engineering water-soluble stand-alone β-sandwich mimetics is a current challenge because of the difficulties associated with tailoring long-range interactions. In this work, single cis-(1R,2S)-2-aminocyclohexanecarboxylic acid mutations were introduced into the edge strands of the eight-stranded β-sandwich mimetic structures from the betabellin family. Temperature-dependent NMR and CD measurements, together with thermodynamic analyses, demonstrated that the modified peripheral strands exhibited an irregular and partially disordered structure but were able to exert sufficient shielding on the hydrophobic core to retain the predominantly β-sandwich structure. Although the frustrated interactions decreased the free energy of unfolding, the temperature of the maximum stabilities increased to or remained at physiologically relevant temperatures. We found that the irregular peripheral strands were able to prevent edge-to-edge association and fibril formation in the aggregation-prone model. These findings establish a β-sandwich stabilization and aggregation inhibition approach, which does not interfere with the pillars of the peptide bond or change the net charge of the peptide.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...