(2-Fluoroallyl)boronates: new reagents for diastereoselective 2-fluoroallylboration of aldehydes†
Abstract
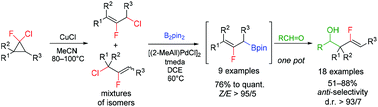
A Pd-catalyzed borylation of 2-fluoroallyl chlorides with B2pin2 in the presence of [(2-MeAll)PdCl]2/TMEDA or [(2-MeAll)Pd(IPr)Cl] was developed to afford (2-fluoroallyl)pinacolboronates with high Z-selectivity. The products proved to be useful for anti-selective 2-fluoroallylboration of aromatic and aliphatic aldehydes.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...