Streamlined chemoenzymatic total synthesis of prioritized ganglioside cancer antigens†
Abstract
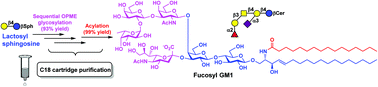
A highly efficient streamlined chemoenzymatic strategy for total synthesis of four prioritized ganglioside cancer antigens GD2, GD3, fucosyl GM1, and GM3 from commercially available lactose and phytosphingosine is demonstrated. Lactosyl sphingosine (LacβSph) was chemically synthesized (on a 13 g scale), subjected to sequential one-pot multienzyme (OPME) glycosylation reactions with facile C18-cartridge purification, followed by improved acylation conditions to form target gangliosides, including fucosyl GM1 which has never been synthesized before.
- This article is part of the themed collections: Chemical Biology in OBC and Celebrating excellence in research: women of organic chemistry


 Please wait while we load your content...
Please wait while we load your content...
