General and stereoselective aminoxylation of biradical titanium(iv) enolates with TEMPO: a detailed study on the effect of the chiral auxiliary†
Abstract
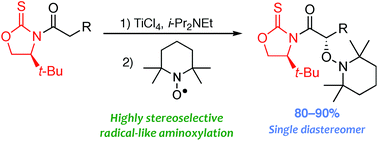
A comprehensive analysis of the influence of the chiral auxiliary on the α-aminoxylation of titanium(IV) enolates with TEMPO indicated that (S) 4-tert-butyl-1-oxazolidine-2-thione is the most appropriate scaffold to provide a single diastereomer in high yields for a variety of substrates, which converts such a radical reaction into a highly chemo- and stereoselective oxidation.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...