Activation of disulfide bond cleavage triggered by hydrophobization and lipophilization of functionalized dihydroasparagusic acid†
Abstract
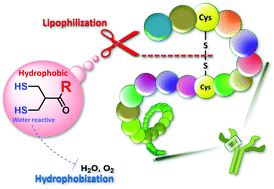
Concisely synthesized and functionalized dihydroasparagusic acid (DHAA) derivatives were used to show that the introduction of a hydrophobic functional group dramatically reduced air oxidation activity at the dithiol moieties and dominantly activated the cleavage of S–S bonds in proteins, presumably due to the hydrophobization and lipophilization. Notably, the reaction sites of water-reactive dithiol moieties behaved similarly to hydrophobic and lipophilic functional groups, which suggests impersonation of the reaction site.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...