C–H alkylation reactions of indoles mediated by Pd(ii) and norbornene: applications and recent developments
Abstract
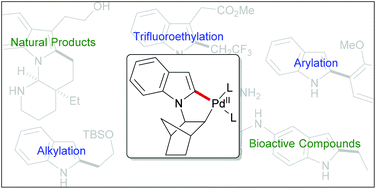
The Catellani reaction enables an ortho-C–H activation based on oxidative addition of Pd(0) and an intermediary carbopalladation of norbornene. Among its variants, the recently developed C2-selective alkylation of indoles is particular as it employs Pd(II) as the source of palladium. This review describes the mechanistic background of this transformation. Applications in total synthesis and in the synthesis of biologically relevant molecules are illustrated and further developments of the method are discussed.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...