Efficient synthesis of ferrocifens and other ferrocenyl-substituted ethylenes via a ‘sulfur approach’†‡
Abstract
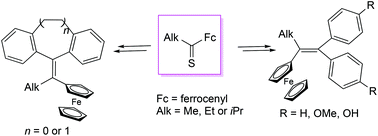
Stable and non-odorous alkyl ferrocenyl thioketones react with bis(4-methoxyphenyl)diazomethane according to the ‘two-fold extrusion’ reaction principles, and tetrasubstituted ethylenes obtained thereby can be demethylated to give (Fc,2OH)-ferrocifens in good yields. The method offers an alternative approach to this class of medically relevant compounds. A similar protocol with alkyl ferrocenyl thioketones and selected diaryldiazomethanes leads to ferrocenyl-substituted ethylenes including dibenzofulvenes. These products are of potential interest for electrochemical and photophysical studies.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...