Transient imines as ‘next generation’ directing groups for the catalytic functionalisation of C–H bonds in a single operation
Abstract
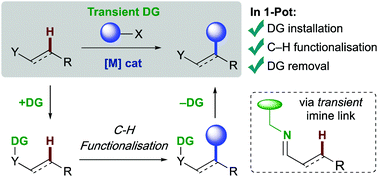
C–H functionalisation promises a paradigm shift in synthetic planning. However, the additional steps often required to install and remove directing groups currently detract from the efficiency. The strategy of reversible installation of a directing group via an imine linkage has recently emerged, with the imine formed and hydrolysed in situ. Such transient directing groups can promote transition metal catalysed functionalisation of unactivated C–H bonds of aldehydes, ketones and amines. This approach removes additional steps usually required for covalent directing groups and can use catalytic quantities of the imine forming component. This review updates the rapidly developing field of transient directing groups for C–H functionalisation on sp2 and sp3 carbon centres, to form new C–C and C–X bonds. We focus on the structures of the transient directing groups as mono or bidentate coordinating groups for various metal catalysts.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...