Copper-catalyzed ambient-temperature decarboxylative annulation of isatins with amidine hydrochlorides: a facile access to 2-(1,3,5-triazin-2-yl)aniline derivatives†
Abstract
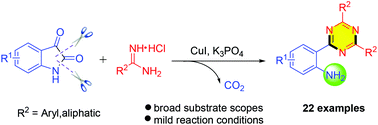
A copper-catalyzed cascade reaction using isatins and amidine hydrochlorides for the synthesis of 2-(1,3,5-triazin-2-yl)aniline derivatives has been developed. This reaction features commercially available starting materials, mild reaction conditions and good functional group tolerance.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...