Cs2CO3-promoted methylene insertion into disulfide bonds using acetone as a methylene source†
Abstract
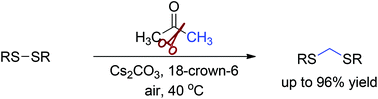
An efficient halogen-free Cs2CO3-promoted methylene insertion into disulfide bonds has been achieved using acetone as a methylene source under mild conditions. This method provides a convenient and practical route to dithioacetals in up to 96% yield with good functional group compatibility.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...