An I2-mediated aerobic oxidative annulation of amidines with tertiary amines via C–H amination/C–N cleavage for the synthesis of 2,4-disubstituted 1,3,5-triazines†
Abstract
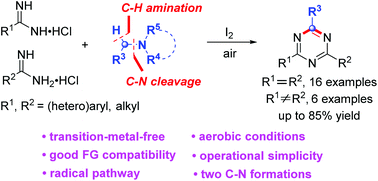
An iodine-mediated formal oxidative cycloaddition of amidines with tertiary amines was first demonstrated in air. Both symmetrical and unsymmetrical 2,4-disubstituted 1,3,5-triazines were obtained in up to 85% yields. It is noted that a tertiary amine was employed as a one carbon synthon of 1,3,5-triazines and two C–N bonds were formed in one pot. Control experiments revealed that the reaction underwent a radical pathway promoted by I+. The method is transition-metal-free, peroxide-free, and operationally simple to implement with a wide scope of substrates.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...