Metal-free α-alkylation of alcohols with para-quinone methides†
Abstract
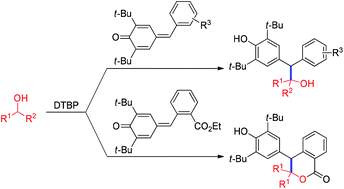
A metal-free α-alkylation of alcohols with para-quinone methides (p-QMs) for accessing alcohol-containing phenols and dihydroisocoumarins has been developed. The alcohols were converted to α-oxy radicals in the presence of di-tert-butyl peroxide (DTBP), which were added to p-QMs for aromatization and C(sp3)–C(sp3) bond formation.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...