Metal-free C(sp2)–H functionalization of azoles: K2CO3/I2-mediated oxidation, imination, and amination†
Abstract
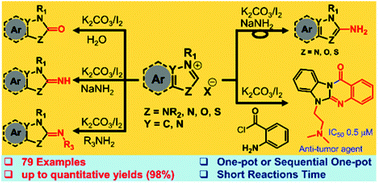
The direct C2−H oxidation and imination of a wide variety of azoles was achieved by using a commercially available simple K2CO3/I2 reagent combination. The iodinated azole adduct, produced via the in situ generation of N-heterocyclic carbene, is the key intermediate for C2−H oxidation, imination, and amination of azoles. Significantly, these reactions proceed under mild conditions with high to excellent yields, are scalable to large quantity and exhibit a broad substrate scope. Interestingly, this direct C2−H imination method allowed us to access various pharmacologically active N6-alkyl or N6-aryl substituted benzimidazoquinazolinone scaffolds through intramolecular C−H imination in a sequential one-pot reaction.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...