Persulfate-promoted oxidative C–N bond coupling of quinoxalinones and NH-sulfoximines†
Abstract
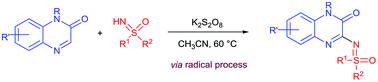
The persulfate-meditated oxidative C–N bond coupling of the C–H bond of quinoxalinones and the N–H bond of NH-sulfoximines is reported. The reaction proceeds smoothly under transition metal-free conditions and provides good to excellent yields of sulfoximidoyl-functionalized quinoxalinone products under mild conditions. The optimized conditions were found to be suitable for a range of sulfoximine and quinoxalinone substrates. This reaction offers a new and convenient strategy to directly install the sulfoximine moiety into the C3 position of quinoxalinone.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...