A metal-free direct C (sp3)–H cyanation reaction with cyanobenziodoxolones†
Abstract
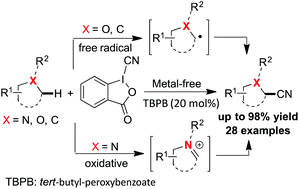
A metal-free protocol of direct C(sp3)–H cyanation with cyanobenziodoxolones functioning as both cyanating reagents and oxidants was developed. Unactivated substrates, such as alkanes, ethers and tertiary amines, were thereby transformed to the corresponding nitriles in moderate to high yields. Mechanistic studies indicated that the cyanation proceeded with two potential pathways, which is highly dependent on the substrates: (1) a free radical case for alkanes and ethers and (2) an oxidative case for tertiary amines.


 Please wait while we load your content...
Please wait while we load your content...