A double-click approach to the protecting group free synthesis of glycoconjugates†
Abstract
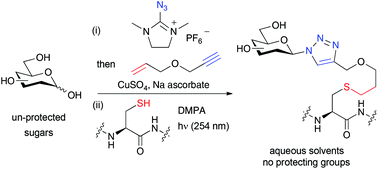
The use of a bi-functional linker, containing an alkyne and an alkene, allows the protecting group free conjugation of reducing sugars to thiols via a double click process. Firstly the linker is attached to the sugar via one-pot glycosyl azide formation and Cu-catalysed azide–alkyne cycloaddition. Photochemical thiol–ene click reaction then allows conjugation to a range of thiols, including cysteine residues of peptides.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...