Copper(ii)-mediated, carbon degradation-based amidation of phenylacetic acids toward N-substituted benzamides†
Abstract
The unprecedented synthesis of N-aryl substituted benzamides via the assembly of primary amines and phenylacetic acids has been developed in the presence of copper(II) acetate. This tandem transformation involving carbon–carbon bond cleavage provides a complementary tool with particular application in the synthesis of secondary benzamides.
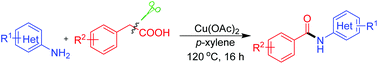
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...