Bis(phenylsulfonyl)methane mediated synthesis of olefins via a halogen elimination and double bond migration†
Abstract
An effective dehydrochlorination of bis(phenylsulfonyl)alkane to prepare alkene building blocks is developed. The elimination together with double bond migration results in a variety of β,γ-unsaturated bis(phenylsulfonyl)olefins in good yields with only E geometry. The following chemical diversification represents an easy and straightforward access to a series of alkene building blocks.
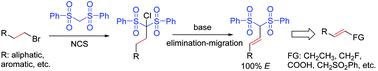
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...