Concise total synthesis of (+)-asperazine A and (+)-pestalazine B†
Abstract
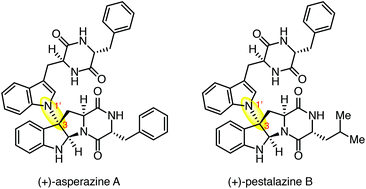
The highly convergent total synthesis of dimeric diketopiperazine alkaloids (+)-asperazine A and (+)-pestalazine B is described. A critical aspect of our expedient route was the development of a directed regio- and diastereoselective C3–N1′ coupling of complex tetracyclic diketopiperazine components. This late-stage heterodimerization reaction was made possible by design of tetracyclic diketopiperazines that allow C3-carbocation coupling of the electrophilic component to the N1′ locus of the nucleophilic fragment. The application of this new coupling reaction to the first total synthesis of (+)-asperazine A led to our revision of the sign and magnitude of the optical rotation for the reported structure.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...