Transition-metal-catalyst-free synthesis of anthranilic acid derivatives by transfer hydrogenative coupling of 2-nitroaryl methanols with alcohols/amines†
Abstract
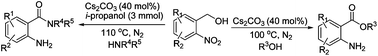
By using a transfer hydrogenative coupling strategy, we herein describe a new method for the efficient synthesis of anthranilic acid derivatives, a significantly important class of compounds with extensive applications in organic synthesis and the discovery of bioactive molecules, from 2-nitroaryl methanols and readily available alcohols/amines. The synthesis proceeds with the merits of no need for a transition metal catalyst, operational simplicity, broad substrate scope, good functional tolerance, and high step efficiency, which offers a useful alternative to access anthranilic acid derivatives.


 Please wait while we load your content...
Please wait while we load your content...